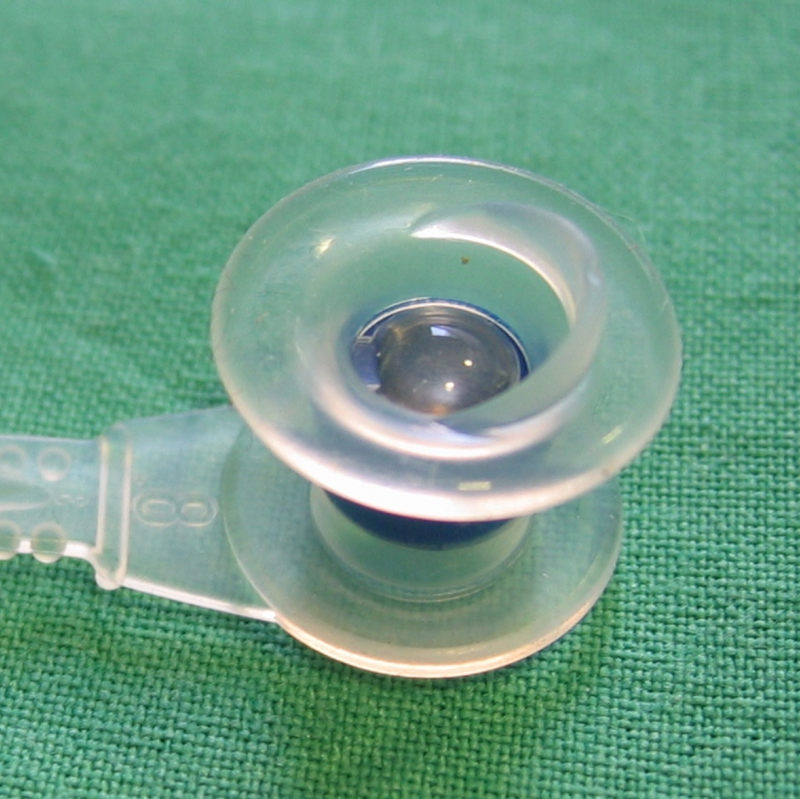
Provox® 2
Clinical application
The Newvox® prosthesis is manufactured by Vygon and marketed mainly in France. It was developed by Professor Traissac. It is hitherto the only polyurethane prosthesis that is said to have a certain level of biofilm resistance. One other special feature is that the length of the shaft is adjustable by sliding the tracheal flange. It is therefore only available in one standard length that is shortened as required.
| Type of prosthesis: | Low-resistance indwelling prosthesis |
|---|---|
| Outer diameter of shaft: | 7.5 mm |
| Shaft lengths: | 4.5 mm, 6 mm, 8 mm, 10 mm, 12.5 mm, 15 mm |
| Material: | silicone |
| Valve type: | flap valve |
| Method of insertion: | anterograde, retrograde if necessary |
| Indication: | standard prosthesis for primary and secondary insertion |
| Biofilm resistance: | no |
| Underpressure stability: | no |
| Non-fatiguing valve mechanism: | no |
| Prevalence: | worldwide |
Literatur
1. A decade of postlaryngectomy vocal rehabilitation in 318 patients: a single Institution's experience with consistent application of provox indwelling voice prostheses.
Op de Coul BM, Hilgers FJ, Balm AJ, Tan IB, van den Hoogen FJ, van Tinteren H.
Arch Otolaryngol Head Neck Surg. 2000 Nov;126(11):1320-8.
2. Multi-institutional assessment of the Provox 2 voice prosthesis.
Ackerstaff AH, Hilgers FJ, Meeuwis CA, van der Velden LA, van den Hoogen FJ, Marres HA, Vreeburg GC, Manni JJ., Arch Otolaryngol Head Neck Surg. 1999 Feb;125(2):167-73.
3. Development and clinical evaluation of a second-generation voice prosthesis (Provox 2), designed for anterograde and retrograde insertion.
Hilgers FJ, Ackerstaff AH, Balm AJ, Tan IB, Aaronson NK, Persson JO., Acta Otolaryngol. 1997 Nov;117(6):889-96.
4. Voice rehabilitation after laryngectomy with the Provox voice prosthesis. Surgical and technical aspects. II], Hilgers FJ, Balm AJ, Gregor RT., HNO. 1995 Apr;43(4):261-7.

Atos Medical GmbH
Mülheimer Straße 3-7
53840 Troisdorf